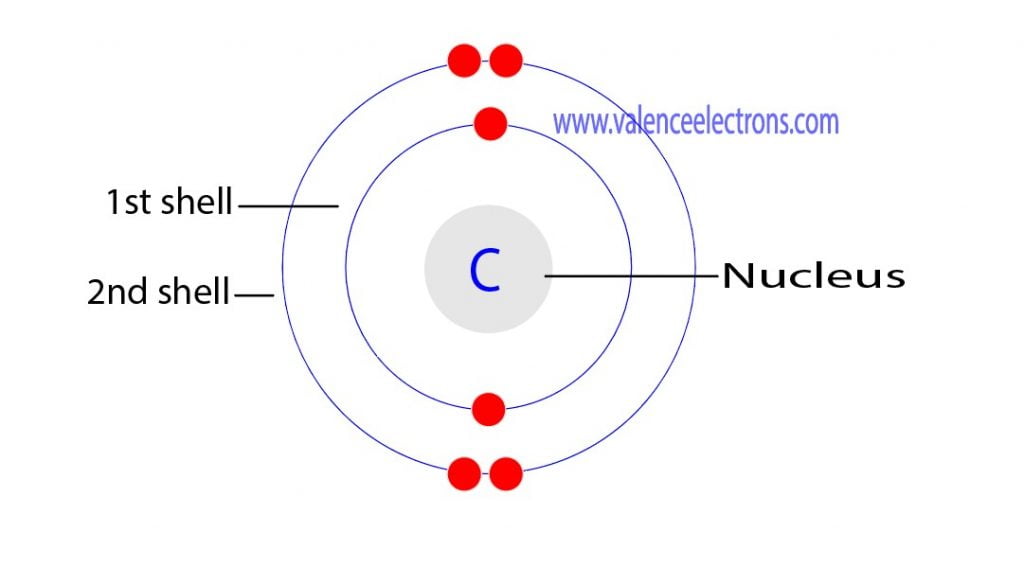
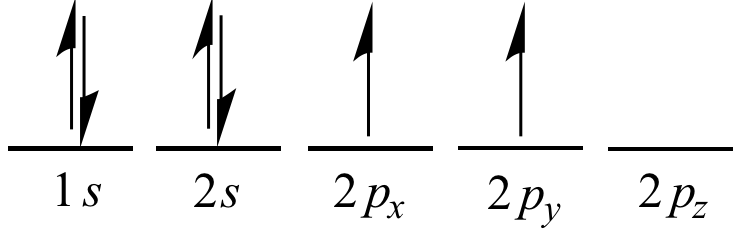![The electron configuration for the carbon atom is: (a) [He] 2s^22p^2 (b) [He] 2s^4 (c) [Ne] 2s^22p^2 (d) 1s^22p^4 (e) None of these | Homework.Study.com The electron configuration for the carbon atom is: (a) [He] 2s^22p^2 (b) [He] 2s^4 (c) [Ne] 2s^22p^2 (d) 1s^22p^4 (e) None of these | Homework.Study.com](https://homework.study.com/cimages/multimages/16/aufbau7621802225308557580.png)
The electron configuration for the carbon atom is: (a) [He] 2s^22p^2 (b) [He] 2s^4 (c) [Ne] 2s^22p^2 (d) 1s^22p^4 (e) None of these | Homework.Study.com
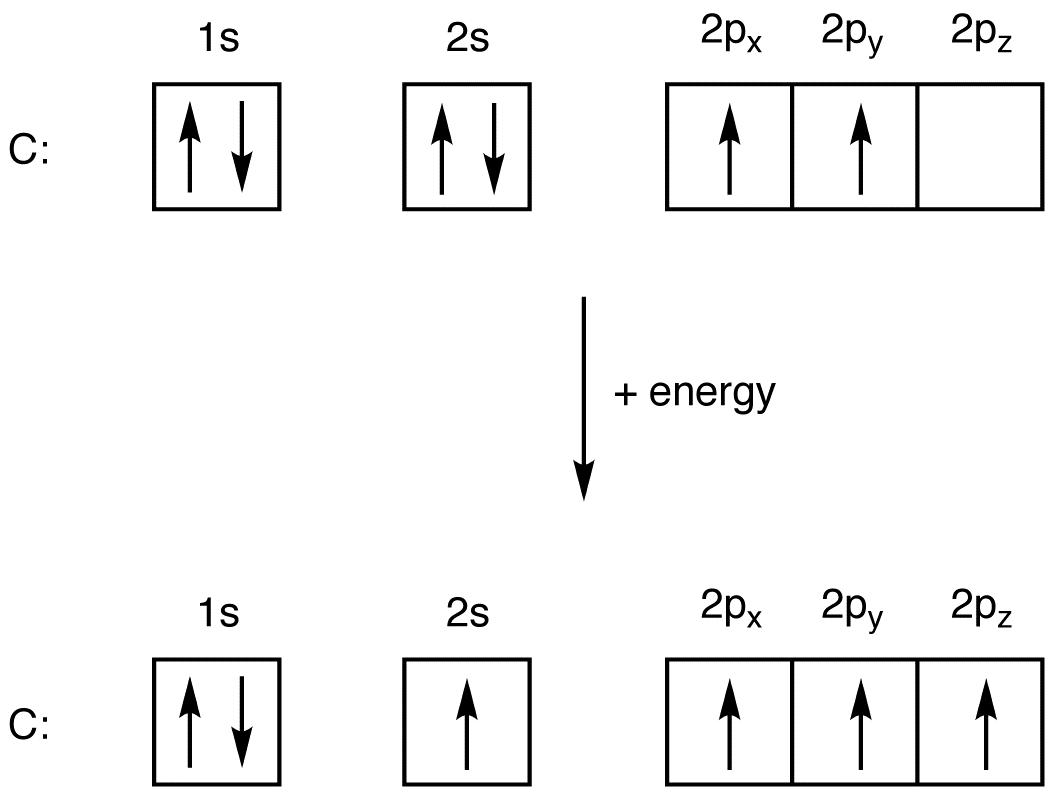
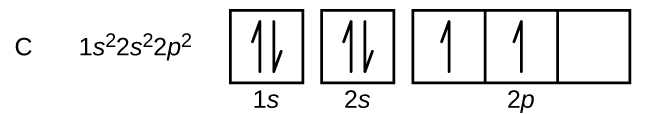
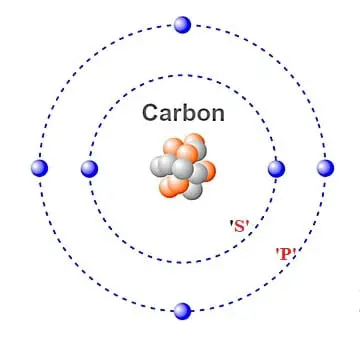
Write the ground state electron configuration for a neutral carbon atom, and for an excited state of carbon? | Socratic
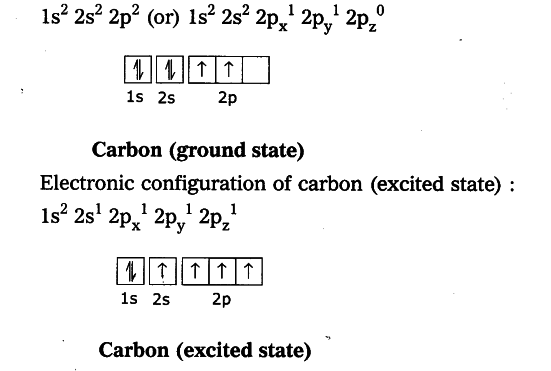
Explain the four unpaired electrons in carbon atom through excited state - CBSE Class 10 Science - Learn CBSE Forum

Ground state electron configurations of boron, carbon, nitrogen and... | Download Scientific Diagram

Write the ground state electron configuration for a neutral carbon atom, and for an excited state of carbon? - CBSE Tuts

Electron configuration Electron shell Valence electron Carbon, carbon, chemical Element, angle png | PNGEgg