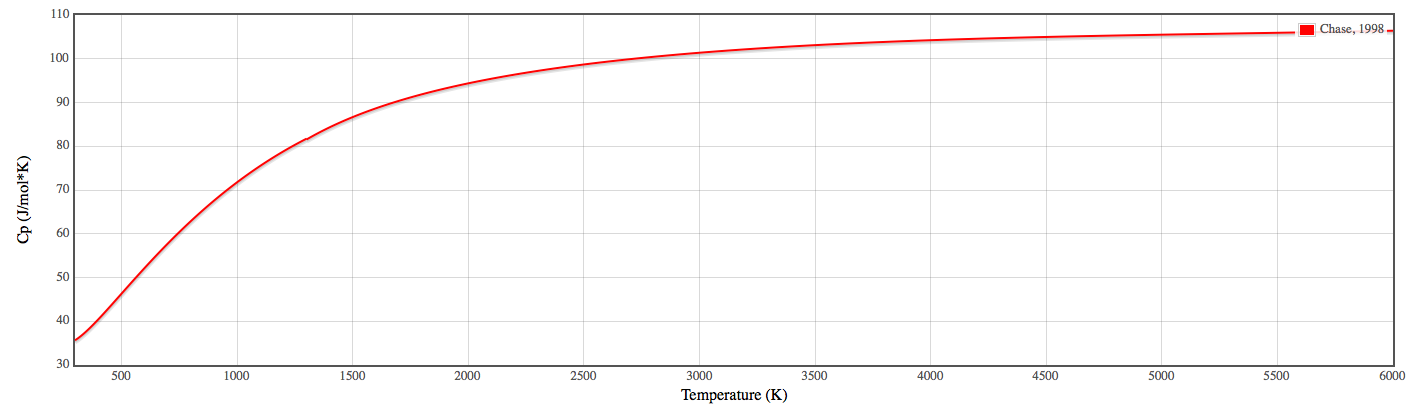
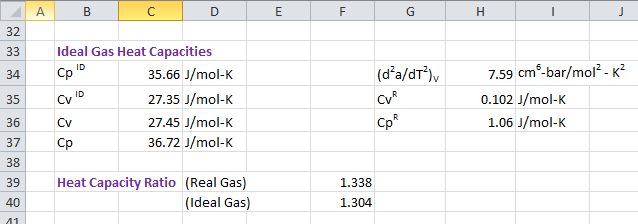
![PDF] Isobaric Heat Capacity Measurements of Liquid Methane + Propane, Methane + Butane, and a Mixed Refrigerant by Differential Scanning Calorimetry at High Pressures and Low Temperatures | Semantic Scholar PDF] Isobaric Heat Capacity Measurements of Liquid Methane + Propane, Methane + Butane, and a Mixed Refrigerant by Differential Scanning Calorimetry at High Pressures and Low Temperatures | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c86887f1b117f3d3174a70149819ff235e70f396/23-Table2-1.png)
PDF] Isobaric Heat Capacity Measurements of Liquid Methane + Propane, Methane + Butane, and a Mixed Refrigerant by Differential Scanning Calorimetry at High Pressures and Low Temperatures | Semantic Scholar

The heat capacity of a bomb calorimeter is 300 J/K . When 0.16 gm of methane was burnt in this calorimeter the temperature rose by 3^∘C . The value of U per

Isobaric specific heat capacity of natural gas as a function of specific gravity, pressure and temperature - ScienceDirect

A sample of 0.16 g CH(4) was subjected to combustion at 27^(@)C in a bomb calorimeter. The temperature of the calorimeter system (including water) was found to rise by 0.5^(@)C. Calculate the
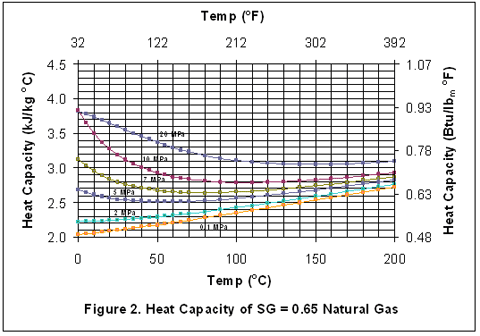
Variation of Natural Gas Heat Capacity with Temperature, Pressure, and Relative Density | Campbell Tip of the Month

Table 1 from The Specific Heats, C σ , and C V , of Compressed and Liquefied Methane. | Semantic Scholar

Thermophysical properties of methane with temperature a) Specific heat... | Download Scientific Diagram

The rotational heat capacity of methane with only the first five terms... | Download Scientific Diagram

0.16 g of methane was subjected to combustion at 27^∘C in a bomb calorimeter. The temperature of the calorimeter system (including water) was found to rise by 0.5^∘C . Calculate the heat
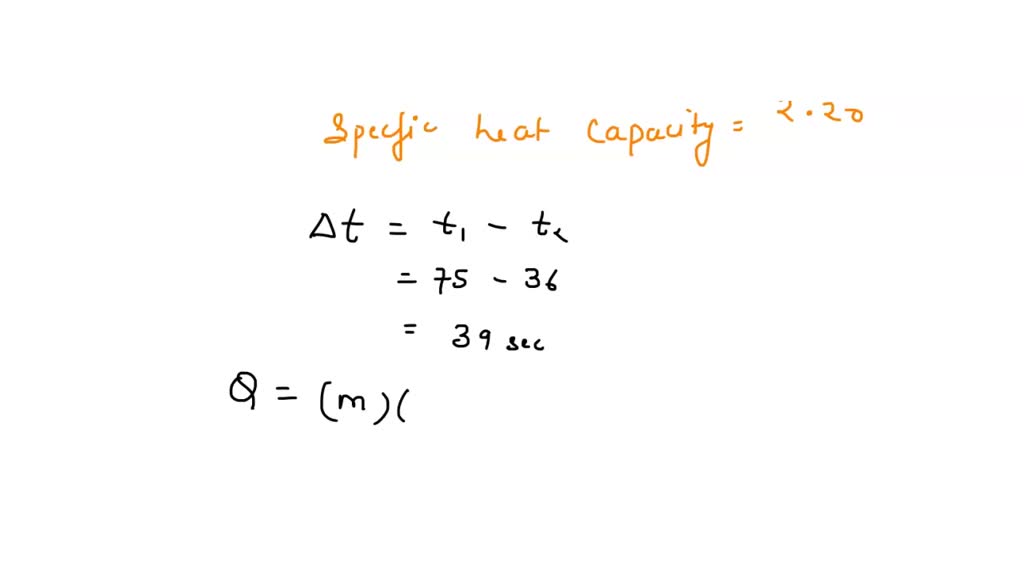
SOLVED: The specific heat capacity of methane gas is 2.20 J/g-K. How many joules of heat areneeded to raise the temperature of 5.00 g of methane from 36.0°C to 75.0°C? a) 88.6
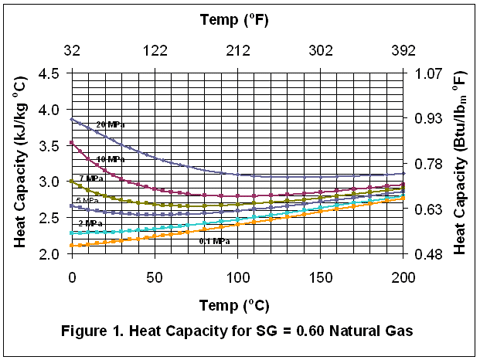
Variation of Natural Gas Heat Capacity with Temperature, Pressure, and Relative Density | Campbell Tip of the Month

Molecular heat capacity of methane/air mixtures with different diluents... | Download Scientific Diagram